Hydrocephalus Care & Treatment Information with Free Forms & mHealth Apps
By: Stephen Dolle, Neuroscientist & CNS Shunt User for Hydrocephalus (since 1992)
Updated: Feb. 28, 2018
Prior to developing hydrocephalus in 1992, I spent 17 years as a nuclear medicine technologist, and 10 yrs of this with my own company, Certified Nuclear Imaging. I regularly performed cisternogram and shuntogram procedures for hydrocephalus, and wrote many protocols at more than 50 hospitals where I provided services. I was very adept both in medical technology, and in clinical medicine. I knew that hydrocephalus shunts were problematic. And in 1992 I became a patient with hydrocephalus after an auto accident, and my life would forever change. I've put this site and web page together over the last 12 years, and I do all of the web site work myself. I have an extensive understanding of hydrocephalus and surgical outcomes, many of the CNS shunts in use today, the various methods of testing that are available, and many issues in living with hydrocephalus. Through this site, you can both learn more about hydrocephalus and many of my efforts, and also how to reach me if you'd like a patient consult. I've got two of my consult reports posted here for prospective patients/families to read over.
As of 2015, I have been mostly writing on my company blog and have a number of blogs on hydrocephalus, mHealth mobile apps, drumming, sensory processing disorder, and cognitive neuroscience topics. In May 2016, I began offering FREE Android downloads ($4.99 value) of the popular Elecont eWeather HD app which I write about for managing weather triggers of migraine & headaches. READ about the apps I recommend for hydrocephalus, such as apps to screen your home & surroundings for magnetic fields, and decibel meter apps to measure sound levels for sensory processing disorder.
In June 2017 I appeared on Ch. 6 TV Laguna Woods, California, to discuss some of his exciting neurosciences discoveries and efforts as a patient advocate, mHealth developer, and drum circle facilitator.
Stephen Dolle June 2017 TV Interview Laguna Woods, CA
DolleCommunications Blog - Home
New: FREE Android Downloads of Elecont eWeather HD Mobile App for Managing Migraine Headache
New: Tips and New Treatments in Managing Sound Sensory Processing Disorder
New: Web Section with tons of info on our efforts in the Cognitive Neurosciences
Below are Free Downloadable Hydrocephalus Monitoring Forms (in PDF files) - Do your own monitoring:
1. DiaCeph NPH Monitoring Form
2. DiaCeph NPH Monitoring Instructions
3. 2-Day Hydrocephalus Monitoring Graph
I am working on a proposal for the DiaCeph mobile app. I will write more later with more information. I also have a fun site for hydrocephalus online now at http://www.HydroPowered.org for sharing art, music, and fun stuff by people impacted by hydrocephalus. And below, there is a link to a Facebook group where you can share and download content.
HydroPowered On our Blog:

Hydrocephalus and NPH Monitoring Consults
Just as shunt manufacturer reps are present in most of your shunt surgeries, you should have pre & post shunt revision/implantation monitoring to assure that you receive the best possible outcome from your hydrocephalus care and treatment.
Hydrocephalus monitoring & consults are based on my DiaCeph Shunt Monitoring System, and my nearly 40 years of working with hydrocephalus. My qualifications include:
1. Nuclear medicine technologist, hydrocephalus testing, imaging consultant 1976 -1992.
2. Researcher/advocate of CNS shunt technology, inventor of DiaCeph shunt monitoring system 1994 - present.
3. FDA patient advocate, FDA petitions, regulatory guidance, 1999 STAMP Conference 1994 - present.
4. Experienced as a patient living with hydrocephalus, 12 shunt revisions, more than 50 brain scans 1992 - present.
5. Hydrocephalus & CNS shunt technology consultant answering inquiries, and providing patient consults from 1996 - present.
6. Neuroscience researcher in CNS shunt technology, sensory processing disorders, assistive cognitive technologies, drumming for the brain, cognitive accessibility, and human factors designs 1998 - present.
I include two patient reports below to illustrate the information I am able to provide through my DiaCeph monitoring and case reviews. I instruct the patient/family how to perform the monitoring on paper forms and instructions, typically over 10 to 14 days. When the monitoring is complete, you return the materials to me, and I plug the data into my computer and generate graphs of your data, then prepare a report of my findings and recommendations which can be used for ongoing care, corrective revision, and/or shunt pressure adjustments. Unfortunately, none of this is covered by insurance.
In the past, shunt monitoring would have involved a 3rd party to provide testing instructions to the patient or family. Someone would need to instruct/train the physician on how to interpret results. The insurance company would then have to pay the physician to process and interpret the results. My current DiaCeph monitoring involves similar paper forms, charts, and instructions as above, only I provide a diagnostic report for you and your physician. If an app were available, you would be doing all of this on your own. However, I suspect myself, your neurosurgeon, or others, may still be involved to make sure monitoring is done correctly. I continue to work on development of this app.
I do both hourly consultations, and hydrocephalus monitoring. The monitoring from beginning to end usually takes 5 - 7 hours, billed at $125.00/hour, making the total cost $625.00 and $875.00. I can also speak to your doctors. But, I do not have staff privileges at any hospitals.
If you are interested in this monitoring, complete the Authorization to Release Health Records (PDF file) and Professional Services Agreement (PDF file), and mail them along with a check for $625.00 (5-hours of my time) to 3908 1/2 River Ave., Newport Beach, CA 92663. You can also pay for consultations thru PayPal.com. Clink on the link below and fill in your information. You have the option of paying with your PayPal account, or a major credit card. Best way to reach me is hydro[at]diaceph[dot]com. If you need to speak with me, send me an email with your phone number and I will call you. As of today, February 28, 2018, I no longer have a separate home office phone.
Pay with PayPal
Below, are two (2) Hydrocephalus Consult Reports: 1) The first report was for an NPH patient where a full in-person consult & DiaCeph monitoring were undertaken. My report includes graphs, instructions, analysis, and recommendations for he and his physicians, 19 pages in total. 2) The second report was for an NPH patient where I reviewed the his CT brain scans and medical history, and evaluated key complaints by email & telephone (no monitoring). My report involved my preparing a special Comparison CT Scans document, and writing my findings and recommendations to two letters: one letter for his records, and 2nd letter to his neurosurgeon asking for a re-evaluation of his complaints and consideration for shunt revision.
I have been given permission to publish these online so that others in hydrocephalus care and treatment might learn from my methods. I've developed specialized methods for evaluating serial CT & MRI brain scans, to maximum the information on ventricular size, aging, and brain atrophy.
Report No. 1: NPH Consult/DiaCeph Monitoring Report (Download PDF file)
Report No. 2: NPH Consult w/ Review of CT Scans (Download PDF file)
HydroPowered.org: Fun Stuff for Hydrocephalus
In 2014, I published a fun site www.HydroPowered.org for persons affected by hydrocephalus to share fun stories, art, music, and designs, and it could be used to help raise money for hydrocephalus. My fundraising goal: $100M. There's also a HydroPowered Facebook Group. Feel free to join and share your fun stuff.

My View on Hydrocephalus
I have hosted this section on hydrocephalus
treatment and CNS shunts since
2003 to
give others affected by hydrocephalus more honest information than they might
otherwise get on hydrocephalus. This has come from my first-hand accounts of
having work with hydrocephalus as a nuclear medicine technologist from 1975 to
1992, my experiences from 12 CNS shunt revisions (as of 2014) since 1992, and
from my own research and other efforts with hydrocephalus since 1992 - that have
been considerable. I was highly recognized in 1999 for helping to organize the
FDA's STAMP Conference that year on Hydrocephalus. And in 1996, I authored the
FDA petition on CNS shunts, which gathered a lot of attention. During the
interim period before the FDA issued their 1998 ruling on my petition, I
designed & patented the DiaCeph Test for home monitoring of hydrocephalus. But
could never raise funding. Then in 2004, I became involved in drumming, music
therapy, and sensory processing disorders or SPDs, common in hydrocephalus. And
in 2012, became involved in "cognitive accessibility," which is intended to
address the many cognitive challenges of individuals with hydrocephalus. In
fact, I believe the U.S. is suffering from a Cognitive Accessibility Crisis, and
I've written about it. Through drumming and my efforts with SPDs, I have done
some extraordinary work and research that has helped my own cognitive, balance &
coordination, well being, and personal confidence. The "trenches" caption
came from my earlier 1995-97 writing on the Hyceph-L list serve. My new section
below on cognitive accessibility, having to do with hydrocephalus and all brain
health, is worth visiting.
Cognitive Accessibility
In another section of this web, I present the patented DiaCeph Test for home monitoring of hydrocephalus. I submitted DiaCeph in 2007 to the Southern California AeA Awards Contest (American Electronics Association) 2007 High Tech Awards, but it did not rise in their categories. I wrote my paper in tandem with this Shunt Technology Perspectives presentation and other works by Dr. Aschoff from the University of Heidelberg, who I met in Washington in 1999.
For those who are interested in using the DiaCeph Test to evaluate your present shunt and status, or help match you with your next shunt, please also see my paper, Shunt Selection Model. I have observed that mind/body "applied kinesiology" or "AK" tests, which utilizes the body's own meridians and nervous system (and is widely used in alternative medicine), seems to help identify failure in CNS shunts, and pick up signs of increased ICP. Here is YouTube video of normalized CSF flow following chiropractic adjustment. Here is a simple Illustration of a VP Shunt Placement.
I always felt the Terminator movie character illustrated the advances that implants and diagnostics could bring to CNS shunts and hydrocephalus. Despite the Terminator character being fictional, it does depict what bioengineering and excellence in science might accomplish. In the "Terminator" series, implant diagnostics and maintenance were pivotal in the movies.
Stephen speaks at Food & Drug Administration Medical Device Event on Medical Social Networking on Oct. 7, 2010
Transcript: http://www.fda.gov/MedicalDevices/NewsEvents/WorkshopsConferences/ucm234031.htm
On October 7, 2010, I spoke as a patient advocate with a medical device implant (CNS shunt for hydrocephalus), an inventor, and communications specialist at the above Food & Drug Administration Town Hall in Irvine, California. In addition, I provided Dr. Jeffrey Shuren, Director of the CDRH, with a Complaint on my recent and unusual malfunction with my Orbis Sigma CNS Shunt. The transcript will be available in late October 2010 via this link.
Much of my presentation addressed the state of affairs of mHealth, Social Networking, and HIT in patient care, which I term "Social HIT." But, I also spoke on my experiences as a patient-user of CNS shunts, that 4 of my 8 shunts had failed from "preventable events" that were NOT reported to FDA. There were also physicians present who spoke critically of the FDA's failed oversight of 510k (substantial equivalence) approved medical devices, which I reiterated I felt had also been a problem in CNS shunts, and had asked FDA to add CNS shunts to new and more stringent "Post Market Surveillance" introduced in 2002. This event was actually intended for the medical device industry. But, as I am an inventor of a medical device, the DiaCeph Test, I am well suited to speak at this meeting. I have a rather unique perspective compared to most at this event as an inventor and patient user of a medical device, as a nuclear medicine imaging technologist/business owner of many years and having worked up over 15,000 patients, and a medical intuitive since 1981. I drew a connection in medical intuitive science to the "placebo effect" that occurs widely in drug and medical device studies. The latter has become enhanced from my efforts with drum circles.
In regards to my speaking on "Social HIT," I believe these technologies can fill a documentation void from use of medical devices in everyday life. Many hydrocephalus sufferers frequent Internet forums and write about problems they are experiencing with their shunts. But, industry does not usually become aware of these issues unless there is a revision with a definitive diagnosis and a serious enough problem for the neurosurgeon to contact the manufacturer or rep. So most of what you experience with your shunts goes undocumented and undetected. With "Social HIT," it offers a variety of ways for manufacturers to learn of issues with their medical devices. FDA is currently working with WebMD in a partnership to design Internet forums on prescription drugs, though I'm informed there will be minimal use in medical devices. Patients want to be well with well functioning devices. And manufacturers want to sell more devices. And to do this, they want to reach out directly to patients. The good thing is that such communications will provide more user feedback on issues. Manufacturers will know more, and know it sooner. Some worry that Social HIT will allow manufacturers more opportunity to mislead patients. But I say not so, because such communications will leave a digital paper trail of sorts. Sites like Facebook then will become new grounds for medical manufacturers to peruse and market to patient users. In addition to Internet sites, I envision mHealth apps for your phone that can collect user experiences or data on your shunt, something my DiaCeph Test could do today. But you'll need to input per a series of questions.
 At left, is an earlier power point slide show on how
Diaceph might function as an app. We're a few years away from shunts that
can deliver wireless data to a mobile device. The "telesensor" concept has
been around for 15 - 20 years, but manufacturers could never get all the bugs
out. That's why we don't see it used much today. So I'll be speaking
tomorrow and hope to leave a lasting impression on FDA and industry.
At left, is an earlier power point slide show on how
Diaceph might function as an app. We're a few years away from shunts that
can deliver wireless data to a mobile device. The "telesensor" concept has
been around for 15 - 20 years, but manufacturers could never get all the bugs
out. That's why we don't see it used much today. So I'll be speaking
tomorrow and hope to leave a lasting impression on FDA and industry.
Sound Sensitivity & Sensory Processing Disorders in Hydrocephalus
The CALM Act bill, HR 1084 (Anna Eshoo, D, CA), has now passed thru Congress. The bill is intended to prevent broadcasters from manipulating the volume levels during television and radio commercials. This practice can trigger neurological sequela, complaints, and behavioral problems in persons with hydrocephalus and many neurological disorders, including, autism. The cause of the ill effects of sudden and unmoderated sound is "sensory integration," and the difficulty these persons have in processing sounds. In 2002, I undertook a study on sound and sensory integration that incorporated a "metronome" to evaluate the brain's response to various audio rhythm patterns. I discovered that some of the ill effects from sound had to do with the lack of "rhythmic" pattern. I speculated, and later confirmed that drumming or "drum circles" can actually help affected individuals in processing sound.
Brain Anatomy & Physiology
CNS (central nervous system) shunts raise many of the same issues that were raised in the "Terminator" movies: independence, special abilities, self-diagnostics and repair, and vulnerabilities. CNS shunt implants have been used to treat the medical condition, hydrocephalus, for the last 50 years. Just as was true with the Terminator's implant, CNS shunt function and performance determines the shunt user's capabilities. The "Terminator" movie illustrated the public's willingness to accept a fictional character with a bioimplant, but in the real world a person living with a brain implant still faces some skepticism and the "taboo" that continues to follow neurological disorders in the U.S.
Many in the hydrocephalus community wonder why this taboo or disparity still exists in public perception. But - I believe it has more to do with longstanding Western beliefs and myths about the brain, and continues today because there hasn't been enough positive advocacy and education to dispel the myth and its stereotypes. Then - you have missed opportunities by the medical community to further treatment because of the inadequacy of funding and commitment.
In terms of progress in shunt technology, in the mid-1990s the Codman company (J&J parent) introduced the first programmable shunt. Though it has helped, CNS shunts still face sizable technological and diagnostic hurdles as a result of their neglect over the last 30 years, compared to other medical devices with greater numbers. I receive a lot of inquiries relating to the programmable Codman Hakim and programmable Medtronic Strata shunt for unintended reprogramming of these shunt's settings. The National Hydrocephalus Foundation (http://www.nhfonline.org) published a survey of user experiences reported by patients and families using programmable shunts. Contact them for the results of that survey. The solutions to the above is a whole other discussion. At present, I waiting to see the next generation of shunt advances, specifically, "programmable siphon control devices," which will enable much more finer control of upright shunt flow.
My hydrocephalus web section also hosts information on key FDA decisions that impacted shunts and hydrocephalus in recent years, downloadable patient monitoring forms, information on alternative therapies, and strategies with artificial intelligence and mobile phone assistive technology for people with affects to their cognitive skills. Whenever possible, I hyperlink discussion points to other pages within this web site, and to sources on the Internet. My information is hosted by and paid for by me. I had also been placed on permanent disability in 1992 as a result of my status and confusion in my care, and I have been working very hard ever since to change this status.
I also feature other relevant neuroscience content, including, artificial intelligence devices, music, art, and drum rhythm therapy. I have included several key Food & Drug Administration developments as they impacted hydrocephalus, CNS shunts, and my efforts at progress. My most informative shunt paper is this Shunt Selection Model, which discusses the testing of CNS shunts, covers many shunts in use today, and compares their specifications, courtesy in part to Dr. Aschoff et. al..
The following link is to a blog hosted by the wife of a man who suffered a traumatic brain injury (TBI), and she writes about how "journaling" can really help brain injured patients and their loved ones in overcoming the stress and disillusionment of their situation. She gives instructions and tips on how to get started on journaling. Her blog is: http://journalafterbraininjury.wordpress.com
My Review of Experiences with the Aesculap (Miethke) proGAV Shunt Valve
On Feb. 14, 2012, my Aesculap proGAV & entire CNS shunt system required revision. This was due in part to my gradual ventricular enlargement, thought due to obstruction of the proGAV valve with debris thought to originate from my 14 year-old ventricular catheter. So on this date my entire shunt system was revised and an Orbis Sigma OSV-II Low Pro valve was put in its place. I had stipulated to my neurosurgeon that he revise me with the standard size OSV-II as I had a more favorable earlier experience with this model. Instead, he revised me with the OSV-II Low Pro valve model, with which I had an unfortunate experience in 2010 of overdrainage followed by reciprocal valve obstruction.
In continuing with my review of the Miethke proGAV shunt valve from Aesculap, implanted in May of 2011, my overall review is mixed. I give it a grade of C+. What might have gotten it a higher grade were if the manufacturer made a programming phone app available to patient users to better the current method of finding the most optimized pressure setting. Today Aesculap has released an iPhone app to help clinicians and nursing staff program the proGAV. However, it has no useful role in enabling the patient user's feedback on their status to that would enable a more expedient determination of an optimized pressure selection. If it were not for the above, its causing headaches and sleep disruptions at night during REM sleep, and Aesculap's not so stellar care and follow-up, I'd give it a B+ to A- grade. And if they enlarged the diameter of its flow chamber a bit, I'd give it an A.
The proGAV's strength truly is its programmability with 20 settings to select from, in increments of 1cm of H2O, and a locking pin to reduce unintended reprogramming. Its ShuntAssistant technology, which regulates CSF outflow and overdrainage during sitting and standing, is quite satisfactory. Its requirement that the neurosurgeon preorder and select from one of six (6) different ShuntAssistant resistance levels, is guesswork w/o a true test protocol, and renders the proGav susceptible to mismatch and the possibility of reoperation to correct a mismatch. In fact, I'm still not convinced after 4 months of DiaCeph Testing and 4 CT scans, that the 20cm model I have is the best one for me. But, its big issue for me, is its apparent flow limitation issue at night during periods of REM sleep. It wakes me up with headache often in the middle of the night, or in early morning, and this seems to cause more complaints that day. I now believe, after 4 months of evaluation and experiences, that this REM sleep issue is due to "shunt insufficiency" from a very fine flow path thru its ball and spring chamber. There would need to be bench test and shunt comparison studies, like those performed by Alfred Aschoff, M.D. at the University of Heidelberg, to confirm this issue. At this juncture, my confidence level with my review and findings is about 80%.
The proGAV received FDA 510(k) approval in November 2010. But separately, the proGAV's two integral components, the ShuntAssistant, and the programmable GAV valve, have been in use for 6 to 10 years. I am the 10th patient of my neurosurgeon to use this shunt valve, and felt he had sufficient favorable results to move forward with its use. I also had become frustrated with QA problems with other available valves. In 2008, less than 8 months after implantation, my Codman Medos valve had to be revised after it could no longer retain its setting. And two different OSVII valves lasted less than one year. So it was time to try something new and different.
The CSF flow path of the programmable GAV valve is a ball and spring mechanism, housed within a titanium enclosure for strength and stability. Ball and spring designs are known to be one of the most reliable and longest lasting of any of the shunt valve designs today. The Codman Medos programmable valve is also a ball and spring design. The GAV features a range of twenty (20) opening pressure settings between 0cm H20 and 20cm H20. These selections are not to be confused with ICP measurements, which are more often measured in mm of H20.
The ShuntAssistant portion of the proGAV is its anti-siphon device, and consists of a small cylinder with a floating ball resting on top, which opens and closes variably to the user's body posture - relative to vertical (sitting and standing) and horizontal (laying down). The ShuntAssistant is located within the proGAV assembly just distal to the valve. It has a linear relationship to postural angle. But its siphon retarding action is weighted more proportionally towards vertical, meaning, at only a 30 degree posture from supine, it has 50% of its siphon retarding action. The ShuntAssistant must be pre-ordered at either 10, 15, 20, 25, 30, or 35 cm H20 resistance levels, and therein lies a challenge, needing to know how much anti-siphon resistance a hydrocephalus user will need, without any test measurements prior to surgery. If the user already has a functioning shunt, an ICP tap in the neurosurgeon's office in different postures could offer this information. But to my knowledge, it is not being done. Even with the 0 - 20cm H20 range afforded by the GAV valve, if the hydrocephalus user's postural outflow needs are dramatically less, or more, than the resistance of the ShuntAssistant model, you won't get a good physiologic fit and could end up with shunt related complaints, and wondering whether to revise to a more appropriate ShuntAssistant model. I say this because after 4 months with my 20cm proGAV model, I am not yet convinced that 20cm of upright resistance was the correct/optimal anti-siphon resistance for me. My proGAV has been adjusted 9 times in trying to determine the most optimized setting, relying on my complaints, DiaCeph monitoring, and CT results. My primary complaint remains the abrupt headaches I continue to experience at night and upon waking in the morning.
What truly sets the proGAV apart from the other programmable valves on the market is its locking pin on the programmable wheel, designed to prevent accidental reprogramming of the valve from magnetic fields up to 3 Tesla. The only other valve to offer this feature is the Sophysa Polaris valve, and this valve does not come with built in siphon control. By comparison, the Codman Medos programmable comes with a range of settings from 30mm H20 to 200mm H20, and this valve does come with a siphon control device termed the SiphonGuard.
Another great feature of the proGav is its Shunt Assistant technology. By contrast, the SiphonGuard operates by alternately opening and closing the valve in response to the patient's hydrostatic pressures. The Medtronic SCD, used in both Delta and Strata valves, is normally open to flow and then closes in response to a drop in hydrostatic pressure. But SCDs function are greatly dependant upon where on the user's head it is placed, and w/o an established protocol to match site location and a desired resistance level to each user's upright outflow needs, Medtronic's SCD valves pose a riskier proposition than these other valves. By contrast, the ShuntAssistant offers a "variable" level of resistance per the user's postural angle via a floating ball inserted into the valve's CSF chamber. But I am not entirely comfortable with selecting from one of six ShuntAssistant models, without having done some type of test for the correct level of upright resistance. After having used all the major anti-siphon shunt systems over the last 19 years, spanning the Medos/SiphonGuard, the Delta/SCD, the Orbis Sigma series, and now the Aesculap/ShuntAssistant, I can speak confidently that the ShuntAssistant offers the most physiologic and "stable" siphon regulation of any of these shunt systems. I did like my earlier OSVII valve, but it became problematic after 9 months and failed.
The Aesculap ShuntAssistant also must be placed vertically in the head, neck, or chest, and sometimes a true location and angle may not be possible. For instance, when placed in the back of the head, when the user is lying down, sitting, or standing, there may not be a true 0 or 90 degree angle, and siphon retarding will be changed accordingly. But with the programmable portion of the valve, adjustments of 1cm of H20 or more can be made for fine-tuning the proGav's overall flow rate and resistance level.
Below, are my CT images beginning on Jan. 17, 2011, where there is enlargement of the ventricles, followed by further enlargement on April 9, 2011, and what appears to be more enlargement on June 8, 2011, despite my May 23, 2011 revision. Then, there appears to be a slight decrease in ventricular enlargement on June 20, 2011, followed by normal size (almost too small) ventricles on July 23, 2011. We felt my ventricles came down in size too quickly, and began to raise the proGav setting over a period of two months, eventually up to 6, and rescanned me on Sept. 15, 2011. Those results indicated my ventricular enlargement had returned, so the proGav was returned to a lower setting of 2.
In hindsight, perhaps one mis-step we made was in not obtaining a CT scan right before surgery so as to have a true baseline. My headache, nausea, memory, and balance symptoms had worsened from April 9, 2011 to May 23, 2011, and I had lost appetite and weight. A CT on the revision date would have likely shown considerably more ventricular enlargement as compared to April 9th, rendering the post op CT of June 8, 2011, an improvement, instead of a worsening as we thought. I was having ongoing headaches upon waking in the a.m. and during the daytime after the proGAV revision, with no improvement in memory, and no apparent improvement on CT compared to April 9th, and requested that the proGAV to be lowered all the way to 0 only a few weeks post op. Despite programmable shunts having been in use since 1999, there still remains some considerable confusion and miscalculation in achieving the most optimal settings, and I remain dissatisfied with industry's inattentiveness to this issue. I also think this lack of attentiveness speaks to the high number of unofficial reports of accidental reprogramming of (Codman & Medtronic) programmable shunts. Of course, with the addition of a locking pin on the proGAV valve, this is unlikely to be an issue.
Under my CT images below, I list each of the proGAV's corresponding settings. It was initially set at 10 during surgery, with no improvement in complaints after one week, I asked that it be lowered to 7, and one week later to 5, then to 3, and finally to 0. During this period, almost every morning I awoke with a substantial headache that I assumed was due to increased ICP and underdrainage. On the June 20, 2011, CT we could finally see some decrease in the dilation of my ventricles, and determined the shunt to be working. But, we were still puzzled by the ongoing awaking a.m. headaches. For this reason, it was temporarily raised to a setting of 2, and then with no relief back down to 0. It remained at 0 for almost a month when the July 23, 2011 scan revealed normal sized ventricles. Because of the relatively short period it took for my ventricles to come back to normal, we raised the proGAV gradually to 6, and back down to 2 after the Sept. 15, 2011 scan showed a return of ventriculomegaly.
My daytime headaches have improved, but my issue still is in awaking in the am with headaches and the role that this plays in exacerbation of my complaints and memory during the daytime. Based on my DiaCeph evaluations, four CT scans since my May 23rd revision, and 9 proGav adjustments since implantation, I don't believe any further adjustment of the valve will overcome these complaints. I have had some improvement in memory, but the a.m. shunt events are complicating my outcome and homeostasis with this device. I have been obtaining data thru DiaCeph monitoring. All indicators point to normal patency and operation of the shunt system, and my conclusion is that, "This is as good as it gets" with the proGav 20cm model. I am dissatisfied with awakening in the middle of the night and morning with headache. I suspect, as I wrote above, that this is due to "shunt insufficiency" from the proGav's ultra-fine flow pathway.
Aesculap's customer & patient support in these experiences have been poor. The rep actually responded once by email that they (Aesculap) have no responsibility to me as a patient! Well, September 2011 is National Hydrocephalus Awareness month with a Congressional caucus in Washington, D.C.. Maybe these questions like what is required of shunt manufacturers, patient/user instructions, and pre-surgical protocols might get answered. Hydrocephalus treatment remains 25 years behind in comparative technological advancement. I know that it won't be the ferry-godmother that makes treatment better. It will be a new commitment to quality, support, and development from industry stake-holders!!!
My CT Images from 2011 and Post proGav Revision of May 23, 2011

 No CT Scan obtained during Revision
No CT Scan obtained during Revision
Jan 17, 2011 OSVII April 9, 2011 OSVII Shunt Revision to proGAV May 23, 2011



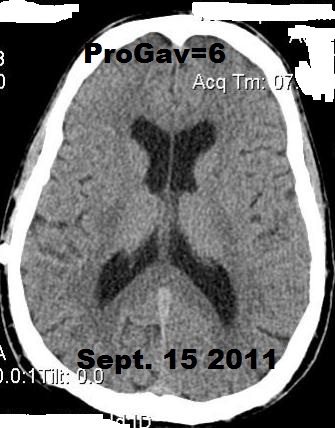
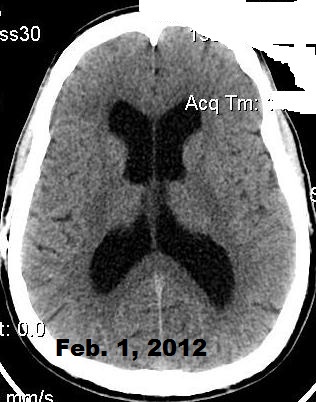
June 8, 2011 proGAV 5/20 June 20, 2011 proGAV 3/20 July 23, 2011 proGAV 0/20 Sept. 15, 2011 proGav 6/20 Feb. 1, 2012 proGav 0/20
Integra's Orbis Sigma (OSVII) Low Pro Valve
I had earlier been been a big supporter of Integra's OSVII shunt valves, but after what I experienced with their new Low Pro valve in February 2010 draining my ventricles down and then causing shunt obstruction within 4 months, I can't support it any more. Their rep shared that Orbis Sigma valves, coming out of manufacturing, have a wide spectrum of flow rates and neurosurgeons should pre-order them with a pre-arranged flow rate in mind. This is poor quality assurance and has caused me to loose my confidence in Integra and the OSV line. In consideration, I am posting this DEVICE WARNING. The issue can lead to overdrainage and subsequent shunt complications. With my 2008 standard OSV-II valve, there was also a definitive difference in ventricular size and headache/cognitive complaints with dietary intake of fats. This may actually be true with many models of shunts, and with a high percentage of persons with hydrocephalus. For me, eating a heavy meal within several hours of bedtime (after 7pm), seemed to cause me to be awakened early in the morning with headaches and increased ICP complaints. Once I tempered my diet, my complaints improved.
My 2010 Low Profile OSV-II shunt was overdraining and causing almost "Slit Ventricles" according to my CT images below. I questioned this new shunt's equivalence and flow rate to the standard Orbis Sigma valve. Integra shrunk the valve's casing, no doubt to win over more use in small children. But the OSV's issue in infants and small children wasn't with the size of the casing. It was the amount of ICP needed to cause sufficient flow thru the valve. Based on my CT scan and complaints, I speculate my Low Pro valve had a 20% higher flow rate than its marketed specification.

CT Image 6-11-2010, 4 months post Low Profile OSV II valve (image/CD courtesy of Hoag Imaging Center)
 CT Image 6-28-2010, 17 days post slit ventricle finding (image/CD
courtesy of Hoag Imaging Center)
CT Image 6-28-2010, 17 days post slit ventricle finding (image/CD
courtesy of Hoag Imaging Center)
It was only back in "October 2008" that my ventricular dilation of 16 years abated. It took 16 years and 7 revisions to get a proper shunt outcome. I typically know my CT results days in advance of my neurosurgeon visit as I obtain it on CD. My health was also helped by research and playing I do with drumming or drum circles. SEE Health & Wellness Benefits to Drum Circles
I continue to believe that in hydrocephalus that neuropsych and headache markers are the most sensitive measure of changes in shunt flow and performance. Neuropsych is the standard with the "Impact Test" for post-concussion monitoring, widely used today in the management of sports concussion. The Impact Test dramatically changed the care and treatment of sports related concussion. The DiaCeph Test method could do the same for hydrocephalus, but won't happen without a groundswell of demand for change. Below, are some of my key CT images of the last 18 years.
Comparison CT Images: Successful 2008 OSV II Fails in Feb. 2010. Revised Low Profile OSV II, Leading to Slit Ventricles

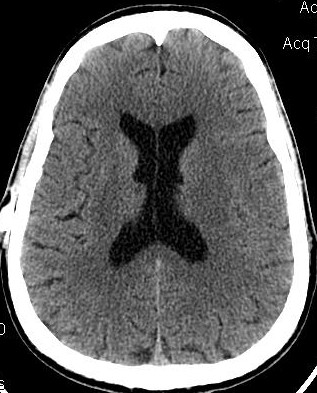
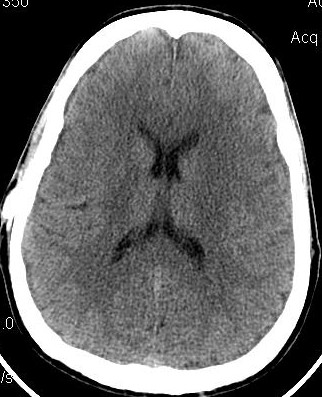

Oct 2008 OSV II June 2009-Fats/Diet Aug 2009 Strict Diet Feb 2010 Shunt Malf Mar 2010 LowPro OSV II June 2010 4mo Post
I


July 1992 pre-shunt Dec 2003 OSV I Nov 2007 Codman Medos SG
Could diet affect your shunt's function and performance? Should you try a low calorie/fat diet?
Maybe. Perhaps more so than maybe. The CT image above far right was taken Oct 2008 and after 6 months on a fairly rigid low fat and low calorie diet, and was stable 5 months later in Feb 2009 (not shown). Then the image below at far left was taken 9 months later in June 2009, after easing up on this diet and amid some returning balance and memory complaints. There is a real measurable increase in ventricular size! The middle image below was taken 3 weeks later as I aggressively resumed an even stricter diet. Then the 3rd image at right below is of 3 weeks later in August 2009 after my strictest low fat and low calorie diet. Notice how this image reveals the smallest ventricle size, albeit after the strictest diet. It would have been nice to have measured ICP and taken CSF samples. I think that if you continue to suffer chronic hydrocephalus complaints, it might be worthwhile to try cutting back on fat and calorie intake.



June 2009 Mod Diet July 2009 Impr Diet Aug 2009 Strict Diet
Hydrocephalus Defined
 Hydrocephalus
is defined as excess cerebral spinal fluid (CSF) accumulation
within any of the four compartments, or ventricles, of the brain. It occurs most
commonly as a congenital condition at birth,
but is also associated
with brain tumors, cysts, trauma, meningitis, adolescent changes, and older age
(NPH). There are about 40,000 new
cases of hydrocephalus diagnosed each year in the United States, with about 70% occurring in young children and newborns.
Eighty percent or more of all new cases are treated with a shunt. Hydrocephalus
remains the leading neurosurgical condition
affecting children today, and can occur at any age, idiopathically, or without
any specific reason. It also occurs in persons
with Parkinson's Disease, Alzheimer's Disease, dementia,
and/or brain atrophy, and hydrocephalus such as NPH (normal pressure
hydrocephalus), are often the most difficult to detect
as they can be masked by other more obvious disorders. Hydrocephalus
is best explained
by examination of normal CSF flow in the brain.
Hydrocephalus
is defined as excess cerebral spinal fluid (CSF) accumulation
within any of the four compartments, or ventricles, of the brain. It occurs most
commonly as a congenital condition at birth,
but is also associated
with brain tumors, cysts, trauma, meningitis, adolescent changes, and older age
(NPH). There are about 40,000 new
cases of hydrocephalus diagnosed each year in the United States, with about 70% occurring in young children and newborns.
Eighty percent or more of all new cases are treated with a shunt. Hydrocephalus
remains the leading neurosurgical condition
affecting children today, and can occur at any age, idiopathically, or without
any specific reason. It also occurs in persons
with Parkinson's Disease, Alzheimer's Disease, dementia,
and/or brain atrophy, and hydrocephalus such as NPH (normal pressure
hydrocephalus), are often the most difficult to detect
as they can be masked by other more obvious disorders. Hydrocephalus
is best explained
by examination of normal CSF flow in the brain.
The brain produces about 20 ml of CSF per hour from the choroid plexus matter located within the lateral ventricles, and circulates this CSF through the third and fourth ventricles and around the surface of the brain. CSF acts to form a hydraulic support system for the brain and spinal chord, and helps move hormones and nutrients throughout the brain. It's more vital function, though, is in the regulation of venous blood pressure in the brain, and consequently ICP (intracranial pressure). This complex regulation impacts the function of higher order cognitive processes. Once circulated through the brain and spinal canal, CSF is reabsorbed via a complex network of tiny vessels called arachnoid villi. When CSF fluid is not reabsorbed at the same rate at which it is produced, due to a blockage (obstructive hydrocephalus) or insufficient absorption (communicating hydrocephalus) - swelling of the ventricles will ensue and exert increased pressure on the vital functions of the brain. MSN features a free interactive illustration of the human brain. At the bottom of this page, we include an MRI image of normal sized ventricles. During all of your care and treatment, it is critically important to have a close friend or relative you can count on - as I'm seen pictured eating with a long time friend above.
The age related form of hydrocephalus termed, normal pressure hydrocephalus, or NPH, occurs mostly in seniors (though can occur as young as 35 years of age). There has been a dramatic rise in NPH over the last several decades as people live longer, and it parallels the rise in dementia, Alzheimer's Disease, and brain atrophy ( seen on brain imaging). This has led to increased difficulties in diagnosing true NPH - known to respond favorably to CNS shunting. Because of its non-specific appearance on brain imaging, and in that NPH symptoms often mimic those of dementia and brain atrophy, NPH has been hard to detect. But it has been receiving more media attention and publicity more recently.
CBS's 60 Minutes II aired a news story "Saved From Senility" in late 2004 that detailed some startling statistics for NPH. The story estimated NPH may affect as many as 1 in every 10 persons with dementia or Alzheimer's Disease, or about 375,000 Americans. In response to this dilemma, Codman & Shurtleff, a neurosurgical division of Johnson & Johnson and maker of CNS shunts, has been airing new TV ads informing seniors of the prevalence of NPH. More recently, spinal tap (pulse wave measurement) and other diagnostic tests are enabling a more accurate diagnosis. My patented Diaceph Test can also aid in the evaluation of NPH, seen in my paper, Shunt Selection Model. Later in this discussion, I identify a simple home screening technique for NPH.
Irrespective of the cause of hydrocephalus, it is treated by either surgical placement of a CNS shunt or by an ETV procedure (endoscopic third ventriculostomy). Here is an educational video of a surgical procedure to place a CNS shunt. CNS shunts divert excess CSF fluid typically to the abdomen (VP shunt), where it is reabsorbed. ETV uses an endoscope to create a permanent new opening in the 3rd ventricle that serves as an alternate pathway for CSF clearance. Treatment by ETV requires that the patient be adequately screened for obstructive hydrocephalus. For other information on hydrocephalus, visit the National Hydrocephalus Foundation or the Hydrocephalus Association.
Both treatments carry risks. If performed successfully, an ETV can last a lifetime - freeing the patient from living with a CNS shunt. CNS shunts typically last about five years on average, and are usually associated with complaints and complex QA issues that affect quality of life. Symptoms of hydrocephalus and that of a malfunctioning shunt include headache, cognitive changes, nausea, vomiting, changes in vision, poor balance or dizziness, malaise, neck pain, precocious puberty and/or stunted growth in children, changes in respiration and heart rate, and coma. Due to the complexities of shunted hydrocephalus, this section is devoted primarily to shunt issues and related complaints. I have also written some discussions on Internet forums. These can be found by a search on Google or Yahoo, typing in "diaceph" in the search window.
Hydrocephalus Treatment with CNS Shunts: A Historical Perspective
 Historical attempts to treat hydrocephalus date back more than 500 years, and
were usually only short term solutions, often ending in death. Some infant
cases did survive without treatment, but the children grew up with very large
heads, and were often marked with developmental disabilities. Over the last few hundred
years, cartoonists and comics have made humor of the image of an abnormally large human
head as you can see from the photo at right.
In
July of 2001, Fox
Sports Net launched a national ad campaign to promote their
sports broadcasts and used this graphic adulteration of a person with a very
large head as an advertising gimmick. Such historical
negative depictions like this ad have served to fan the many misconceptions about
hydrocephalus. I took offense to the ads and initiated correspondence with Fox
and its media carriers to pull the ads, explaining how they were degrading to persons
with hydrocephalus. I turned it over to the Hydrocephalus Association,
who promised to keep me in the chain of
correspondence until it was resolved. Eventually, Fox did discontinue the ads. The Hydrocephalus Association never shared
any specifics on what was reached.
Historical attempts to treat hydrocephalus date back more than 500 years, and
were usually only short term solutions, often ending in death. Some infant
cases did survive without treatment, but the children grew up with very large
heads, and were often marked with developmental disabilities. Over the last few hundred
years, cartoonists and comics have made humor of the image of an abnormally large human
head as you can see from the photo at right.
In
July of 2001, Fox
Sports Net launched a national ad campaign to promote their
sports broadcasts and used this graphic adulteration of a person with a very
large head as an advertising gimmick. Such historical
negative depictions like this ad have served to fan the many misconceptions about
hydrocephalus. I took offense to the ads and initiated correspondence with Fox
and its media carriers to pull the ads, explaining how they were degrading to persons
with hydrocephalus. I turned it over to the Hydrocephalus Association,
who promised to keep me in the chain of
correspondence until it was resolved. Eventually, Fox did discontinue the ads. The Hydrocephalus Association never shared
any specifics on what was reached.
Though CNS shunt designs appear on their surface to be simple technology, manufacture and selecting the most physiological shunt for each patient continues to pose significant challenges to the medical device industry and the field of neurosurgery. The U.S.'s failure to modernize CNS shunt technology speaks volumes about U.S. medical innovation and its over-reliance on Wall Street and for-profit prerogatives in health care, notwithstanding the adverse impact of outdated and burdensome U.S. Food and Drug Administration (FDA) policies.
The person most credited with advancing the treatment of hydrocephalus in the 20th Century was Mr. John Holter, a machinist who in the mid-1950s had a young son who was dying from hydrocephalus, and without any available treatment. Mr. Holter turned his kitchen into a laboratory and produced the 1st silicone shunt designs - all prior to the 1976 regulatory involvement of the U.S. Food and Drug Administration. From the 1960s to the mid-1990s, various shunts have been introduced by U.S. and Western European interests. Europe today seems to play a larger role in the introduction of new shunts. Recent advances in shunts have included externally programmable shunts, auto-regulating shunts, and siphon control devices.
 When crediting the
advances that furthered the treatment of hydrocephalus, one needs to
recognize the special contributions of non-field
individuals. The first being Mr. John Holter above. Next, is (musical group)
The Beatles' EMI records and engineer
Godfrey Hounsfield.
SEE the cool tribute photo art at left created from one of my MRI brain scans,
and edited with my Pic Say app program. After that, I have
been recognized for my contributions after receiving a shunt following a 1992 auto accident. All brought new vision and
their own niche to advances
in care and treatment. John Holter pioneered
the first "silicone shunts," and created several popular shunt designs. EMI Records
funded the
invention of the EMI or 1st CT brain scanner that made the diagnosis of hydrocephalus
and many other disorders then possible. I undertook extensive FDA efforts, introduced the first home monitoring
test for CNS shunts, the DiaCeph Test, and have authored papers on shunts,
assistive technologies, and alternative therapies for the brain.
When crediting the
advances that furthered the treatment of hydrocephalus, one needs to
recognize the special contributions of non-field
individuals. The first being Mr. John Holter above. Next, is (musical group)
The Beatles' EMI records and engineer
Godfrey Hounsfield.
SEE the cool tribute photo art at left created from one of my MRI brain scans,
and edited with my Pic Say app program. After that, I have
been recognized for my contributions after receiving a shunt following a 1992 auto accident. All brought new vision and
their own niche to advances
in care and treatment. John Holter pioneered
the first "silicone shunts," and created several popular shunt designs. EMI Records
funded the
invention of the EMI or 1st CT brain scanner that made the diagnosis of hydrocephalus
and many other disorders then possible. I undertook extensive FDA efforts, introduced the first home monitoring
test for CNS shunts, the DiaCeph Test, and have authored papers on shunts,
assistive technologies, and alternative therapies for the brain.
The first implantable diagnostic device to monitor CNS shunt function was introduced in the late 1980s by Radionics, Inc., now a division of Integra Life Sciences, and termed the "telesensor." The device lent some in-office capability to shunt assessment (provided the patient was implanted with the special device, and the physician purchased the costly reading device). Yet, it faced significant problems with reliability, and could not measure negative intracranial pressure (ICP). Other monitoring attempts include the use of ultrasound to measure CSF flow through shunt catheters, and Cine MRI, a software (pulse wave measurement) addition to an MRI exam. None of the above found broad acceptance in the evaluation of shunt malfunction and performance. Standard testing today continues to be CT and MRI imaging, neurological exam, shunt tap measurements of ICP and shunt patency, isotope clearance imaging, in-hospital ICP monitoring, and coupled ICP/CSF pulse wave monitoring.
The most challenging issue facing the use of CNS shunts continues to be in determining if and where a shunt may not be working properly, termed a shunt malfunction. The next issue is in determining the best matched shunt system for a particular patient prior to surgical placement or revision, and determining the best opening pressure in patients with programmable shunts. Thirdly, chronic neurological changes in the brain due to long term hydrocephalus, such as inflammation of the hippocampus, can manifest and mask as shunt malfunction or pressure mismatch, misleading the neurosurgeon to mistakenly revise or reprogram a shunt. Chronic neurological changes such as this must be evaluated separately using PET (positron emission tomography), fMRI (functional MRI), and/or neuropsychological testing.
It is understood that no shunt is problem free, nor do any designs yet replicate the brain's elaborate physiological ICP auto-regulation mechanism. But, as patient users, we should demand that the field tap into all available resources and work to obtain the best possible availability and adoption of new shunts, and priorities in shunting outcomes.
1996 FDA Petition Leads to Invention of the DiaCeph Test
 The photo at right was taken one day after my 1997, and a few months after I had completed the design
for my DiaCeph Test, which I used to help direct my testing before surgery. I
became shunted after
an auto accident in 1992, and within several years came to learn that my ongoing
and unresolved hydrocephalus complaints
were likely due to poorly understood site placement issues with Medtronic PS Medical's
popular Delta
shunt. The Delta shunt
incorporates an SCD (siphon control device), where Heyer-Schulte's equivalent and sister shunt
incorporates an ASD (anti-siphon device). What led to
my getting really involved in CNS shunt devices from the beginning, was my poor outcome after
three shunt revisions between 1992 - 1993 (all Delta shunts). I had 17 years of medical imaging experience with CNS
shunts. None of my neurosurgeons could
explain my ongoing complaints. In 1995, I came across a series of studies in the
literature (Rekate, Higashi, Drake) that ascribed my complaints to little known issues with anti-siphon
shunts, and in time I was becoming quite knowledgeable on these topics. With previous research experience in the biosciences and
in technology, and having been an accomplished nuclear medicine imaging
specialist and business owner,
I had the background to possibly become an expert in this area, notwithstanding the
challenges that having hydrocephalus was posing to me.
The photo at right was taken one day after my 1997, and a few months after I had completed the design
for my DiaCeph Test, which I used to help direct my testing before surgery. I
became shunted after
an auto accident in 1992, and within several years came to learn that my ongoing
and unresolved hydrocephalus complaints
were likely due to poorly understood site placement issues with Medtronic PS Medical's
popular Delta
shunt. The Delta shunt
incorporates an SCD (siphon control device), where Heyer-Schulte's equivalent and sister shunt
incorporates an ASD (anti-siphon device). What led to
my getting really involved in CNS shunt devices from the beginning, was my poor outcome after
three shunt revisions between 1992 - 1993 (all Delta shunts). I had 17 years of medical imaging experience with CNS
shunts. None of my neurosurgeons could
explain my ongoing complaints. In 1995, I came across a series of studies in the
literature (Rekate, Higashi, Drake) that ascribed my complaints to little known issues with anti-siphon
shunts, and in time I was becoming quite knowledgeable on these topics. With previous research experience in the biosciences and
in technology, and having been an accomplished nuclear medicine imaging
specialist and business owner,
I had the background to possibly become an expert in this area, notwithstanding the
challenges that having hydrocephalus was posing to me.
I reviewed 30 years of published studies and "Freedom of Information" (FOI) documents, and eventually became intrigued with an April 1996 Journal of Neurosurgery study by Higashi, et. al. out of Japan. This Japanese neurosurgery center demonstrated how state-of-the-art engineering and laboratory studies could advance the understanding of CNS shunts. In addition, Higashi cited the "need" for a new type of shunt test that could identify the mysterious malfunctions that were occurring in anti-siphon shunts (ASDs and SCDs). The fact that I was not able to receive proper corrective surgery over a period of 5 years due to limitations in diagnostic testing and understanding of shunts, inspired me to pioneer a test of my own. The critical issue raised by Higashi et. al. with the anti-siphon devices was what they termed, "functional obstructions," where a shunt malfunction occurs due to the device's own internal design, which in this instance, also occurred mostly undetected through "false negative" findings on numerous standardized tests for shunt malfunction. There was be no way of knowing without exploratory surgery, whether the anti-siphon shunt or other component was the source of the patient's ills, and whether shunt revision would resolve the problem. In their study, Higashi and colleagues cited the need for a new specialized test to evaluate these complex malfunctions.
I eventually authored this major Petition to the FDA on Anti-Siphon Shunts to resolve these issues with anti-siphon devices. In an Addendum to my petition, I informed FDA (page 5 and 6) of my new diagnostic test to specifically address SCD and ASD malfunctions. I continued to maintain ongoing communications with Janine Morris, Dr. Anita Kedas, and other key FDA staff on issues relating to CNS shunts, and continued to obtain FOI documents so I could make specific recommendations to FDA. After reading numerous patient posts by shunt users and families on the University of Toronto's HYCEPH-L listserv regarding the unavailability of useful shunt malfunction testing, I broadened my research to encompass "all" shunt concerns, and I determined that 24/7 home shunt monitoring must be a priority. I also learned that without available and adequate diagnostic tests, many patients in Canada and the U.S. were being denied corrective surgery, and some were even being referred for psychiatric evaluations after their physician could not identify the cause of their complaints. This was/is a sad commentary on the status of hydrocephalus.
DiaCeph Test goes to Washington, D.C. for STAMP Conference
 In
late 1997, I finalized my design and algorithms
for the DiaCeph Test
and notified FDA. As an AI (artificial intelligence) type application, DiaCeph non-invasively captures a snapshot of a shunt user's status at any
point in
time. In
February of 1998, with months of DiaCeph trials of monitoring my own status, I guided my
own surgical
revision at Los Angeles Children's Hospital. I later learned my Delta shunt
placement site was actually a "misalignment," and now contraindicated
by Medtronic's Technical Bulletins. Page 6 of the Bulletin illustrates how a Delta or
Strata shunt's flow will be reduced to 5 ml/hour due to misalignment. CSF
flow can also be affected by overlying scalp pressure and scar encapsulation. Diagnostic tests from
1992 to 1997 failed to diagnose my underdrainage and shunt site issues.
In
late 1997, I finalized my design and algorithms
for the DiaCeph Test
and notified FDA. As an AI (artificial intelligence) type application, DiaCeph non-invasively captures a snapshot of a shunt user's status at any
point in
time. In
February of 1998, with months of DiaCeph trials of monitoring my own status, I guided my
own surgical
revision at Los Angeles Children's Hospital. I later learned my Delta shunt
placement site was actually a "misalignment," and now contraindicated
by Medtronic's Technical Bulletins. Page 6 of the Bulletin illustrates how a Delta or
Strata shunt's flow will be reduced to 5 ml/hour due to misalignment. CSF
flow can also be affected by overlying scalp pressure and scar encapsulation. Diagnostic tests from
1992 to 1997 failed to diagnose my underdrainage and shunt site issues.
On September 18, 1998, the FDA granted my FDA Petition on Anti-Siphon Shunts. In their "Ruling," FDA stated they would hold a special conference to address the issues cited in the Petition and Ruling. This conference was subsequently termed the International STAMP Conference, and was held January 1999 in Washington, D.C.. STAMP was the first of its kind FDA effort to try to address quality assurance ( QA) issues and patient outcomes in technology regulated by the FDA, and they said they chose CNS shunts because of my efforts. I also notified FDA with this Petition Ruling Correction, and Notice of Completed Design on DiaCeph Test.
STAMP was supposed to feature presentations on technology, such as ICP
telesensors, new technology prospects, and discuss any anticipated new
technology,
test systems (i.e. DiaCeph Test), research, and proposals for the care of hydrocephalus. It was
to draft recommendations on
research
priorities, better FDA oversight, and prepare first time device literature for
patients. It seemed logical in view of patient literature
provided for prescription drugs, and literature widely provided on electronic devices, appliances, and automobiles. As of
January 2008, no such patient-user literature has ever been made available on CNS
shunts.
In preparation for STAMP, I authored a Paper of Recommendations, where I did not reference my Petition or FDA ruling. I made (50) copies of the paper available at STAMP on a conference table. My efforts were acknowledged by several doctoral members of the STAMP Committee. The FDA's conference leadership made an unprecedented decision to not permit my research or presentation of the DiaCeph Test at STAMP. Instead, Emily Fudge, of the Hydrocephalus Association, spoke and presented a patient survey.
STAMP Conference and CDRH division head, Larry Kessler, Ph.D., would later deny my request to have my Paper of Recommendations be included in the New Technology Section or Executive Summary of the STAMP Conference. The following document is Dolle's STAMP Request to Larry Kessler, Ph.D., and the next document is Larry Kessler's Written Response to Dolle's Request. I also provided insightful Follow-up Recommendations to Janine Morris, STAMP Chair as a roadmap so that FDA might remain committed to improvements in the care of hydrocephalus, CNS shunts, prospective drugs, and new technology.
In 2002, I learned of new "Post Market Surveillance" (PS) being considered by the Food and Drug Administration, on a product by product basis, and studied the FDA's language released in this Code of Federal Regulations. I felt this new PS would be helpful in the routine use and outcomes with CNS shunts, and wrote the following Letter to the FDA for PS Consideration of CNS Shunts.
In light of the more extensive filing requirements, clinical studies, and PS required of prescription drugs, shunt technology undergoes very limited scrutiny as to its outcomes in patients. In the case of a prescription drug, the patient can simply "stop taking' the medication, and can end or minimize any potential adverse effects. But in the case of a CNS shunt, an "intervention" to resolve the shunt issue more often requires major surgery in the form of a shunt revision to remedy the problem. In some cases, a shunt's opening pressure can be non-invasively changed (programmable shunt). As you can read from the FDA's Response to New PS on CNS Shunts, they viewed it differently, and denied my request. Today, perhaps one of the most problematic issues with CNS shunts is in the unavailability of real-time diagnostics on its status, and where possible, a deployment of certain simple interventions by patient/family can help re-establish shunt function. This had been one of my original intents of the DiaCeph design in 1997. Until CNS shunts are improved, we must learn to better use the interventional means available today.
These past FDA failures are raised in light of the NIH's special one-day conference last September 2005, entitled, "Hydrocephalus: Myths, New Facts, and Clear Directions," held in Bethesda, MD. According to NIH stipulations, only "non-profit" organizations were supposed to attend. However, I later learned there were corporations and shunt manufacturers in attendance. The NIH conference took place at the identical building, and was remarkably similar to the STAMP Conference.
NIH gives DiaCeph Two Thumbs Up
In 1998, the DiaCeph Test attracted the interests of a long time neurosurgeon, Eldon Foltz, M.D., at the University of California at Irvine (UCI). Dr. Foltz helped me set up an advisory board, and brought in several field consultants. With a little financial backing and guidance, a corporation was formed and the project was moving forward. The DiaCeph Test was reviewed by staff of the National Institutes of Health (NIH) as part of a University of California technology conference. NIH was excited to fund this project on patient information technology, and cautioned me to secure an accredited scientist to write the NIH grant applications for development costs at the University of California. The University of California had a policy prohibiting university staff from writing such grant applications where the research and technology was not conceived by the university.
 In
2005-06, there were several joint efforts by NIH and the Hydrocephalus Association
said on behalf of those of us
with hydrocephalus. !n 1999, I had seen similar efforts from the Food and Drug
Administration and STAMP
Conference fall to the wayside. There is also often too much emphasis on big
business and organizations. In
2003, the Los Angeles Times reported on an undercover
investigation of wide-scale abuses of non-profit organizations, and the
exorbitant fees paid for celebrity appearances and endorsements. I responded with
a "Letter to the Editor."
In
2005-06, there were several joint efforts by NIH and the Hydrocephalus Association
said on behalf of those of us
with hydrocephalus. !n 1999, I had seen similar efforts from the Food and Drug
Administration and STAMP
Conference fall to the wayside. There is also often too much emphasis on big
business and organizations. In
2003, the Los Angeles Times reported on an undercover
investigation of wide-scale abuses of non-profit organizations, and the
exorbitant fees paid for celebrity appearances and endorsements. I responded with
a "Letter to the Editor."
Following the STAMP Conference in 1999, I was featured in an Orange County Business Journal story for my efforts with the STAMP Conference and DiaCeph Test. A patent application had been filed and later issued, and the project was helped along with support from the late W .L. Dolle, Jr.. Patent representation was provided by the prestigious West Coast firm of Knobbe Martens Olsen & Bear.
Currently, paper forms and user instructions comprise the data collection method of the DiaCeph Test. It is suggested that monitoring be coordinated with any instructions from the treating physician. The forms can be used in tandem with Diamox for screening of NPH, in-office ICP taps, and other diagnostic tests. Diamox is used both as a diagnostic intervention, and therapeutically to reduce CSF production and ICP. A typical use of these forms would entail one to three weeks of baseline monitoring (when malfunction is NOT suspected), followed by a few days to weeks of suspected malfunction monitoring. A prescribed dose of Diamox and simultaneous monitoring may also be incorporated. An improvement following Diamox is suggestive of a diagnosis of hydrocephalus, but this method assumes that cerebral blood flow (CBF) is within normal limits. Often in many seniors suspected of NPH, there will be some compromise in CBF that renders the Diamox test unreliable as a false negative.
Medtronic Strata and Codman Medos Programmable Shunts
Earlier in 2005, I authored the paper, Shunt Selection Model, that evaluated specifications of many leading shunts, and proposed solutions to improve their use. As part of this paper, I wrote to Medtronic PS Medical and asked if they would provide a new "pre-surgical placement protocol" for their Strata and Delta shunts to help neurosurgeons better place them at the correct "anti-siphon" position. Medtronic acknowledged the critical surgical site issue, yet one month later denied this and misled my neurosurgeon into believing he could disregard the Strata's critical surgical implantation instructions, which led to my cancelling the revision. This Strata issue has no doubt affected thousands of patients.
The Delta and Strata Technical Bulletin provides a graph of flow rate vs. zero point for achieving correct site placement. But, as I have learned, few neurosurgeons make any site consideration for how/where the Delta/Strata are placed. They merely place it immediately adjacent to the ventricular catheter, and in many instances, the location ends up contraindicated by Medtronic's own labeling and warnings, with substantial numbers taken back to surgery. Medtronic refused my request for a "placement protocol" to assure proper use of their Strata.
In March of 2007, I contacted the Food & Drug Administration (FDA) and notified them that Medtronic/PS Medical had been misleading neurosurgeons as to the proper placement and use of its Strata shunt. Its site placement graph and minimally worded precaution in its labeling appears to present some confusion for user neurosurgeons. I am concerned with the broader impact it is having on hydrocephalus and shunt use, that it might complicate the adoption of newer and improved shunt technologies.
After being implanted with the programmable Codman Medos shunt in 2007, and observing that it was loosing its setting on eight or so occasions in a few month period, I telephoned my neurosurgeon's office and was told to come in and have it reprogrammed. It was DiaCeph monitoring that initially picked up that my Medos may have jumped to a higher setting. I looked into this accidental reprogramming and found a number of published studies on the topic - that were mostly inconclusive, however, yet raised the substantive possibility of a broader vulnerability problem from household magnets and fields. According to one study, the Sophysa Polaris shunt was the least affected and unlikely to be tripped by household magnets, whereas, both the Strata and Medos could be tripped by appliances around the home.
With my shunt repeatedly loosing its setting, and prior to my revision to the Orbis Sigma Valve, I wrote to Codman and proposed a solution in the form of an instructional video and sports compass, where shunt users could watch a video and use a standardized $12 compass to screen their home and routine for threshold magnetic fields that might reset their shunt. Codman chose not to respond.
If we in the hydrocephalus community, neurosurgeons, patients, family members, and scientists collectively speak up against what we learn to be "adverse and avoidable compromises" in our care, we will see the kind of progress and infusion of new technology that will take your breath away.
My Programming Technique for Codman Medos & Strata Programmable Shunts
Of interest to Codman Medos (Hakim), Strata, and Sophysa programmable shunt users is this new programming method I authored after my experiences with the Medos shunt. Typically in surgery, the neurosurgeon will set the valve to a setting somewhere between 100 and 140, a mid to upper range setting, to avoid any problems with severe overdrainage and subdural hematoma. Later in the office, the setting is then lowered or raised, mostly from a brief neurologic exam and feedback from the patient. If that first adjustment doesn't help, it typically will be dialed in the opposite direction. Codman claims that neurosurgeons are able to find the best setting for most patients within two attempts after surgery. However, I don't see how they can find the most physiologic setting of 18 settings with this method. I believe you dial in the lowest setting possible, then raise it from there until overdrainage is minimized, and you're OK while sleeping at night. Click on the above link to read the full description.
Living with Hydrocephalus: Staying Active in Spite of Limitations
 Having grown up playing piano and touring as a singer, and doing film and
theatre in my adult years, I had a
Having grown up playing piano and touring as a singer, and doing film and
theatre in my adult years, I had a lifetime of experiences in music and
entertainment. In fact, at the time of my brain injury and onset of
hydrocephalus in 1992, I was leaving my medical imaging business to work full
time as a sports & entertainment promoter. When I got hurt, I was about to begin
a Planet Hollywood Restaurant project where I would have met Arnold Swarzenagger,
Bruce Willice, and others from Hollywood.
lifetime of experiences in music and
entertainment. In fact, at the time of my brain injury and onset of
hydrocephalus in 1992, I was leaving my medical imaging business to work full
time as a sports & entertainment promoter. When I got hurt, I was about to begin
a Planet Hollywood Restaurant project where I would have met Arnold Swarzenagger,
Bruce Willice, and others from Hollywood.
After my onset of hydrocephalus, I began to use music as "therapy" in a number of ways. My memory was terrible, so when I began to play drums, I found that I could play from feel and instinct, rather than recalling pre-pared rhythm parts, and I fairly quickly began to excel. Learning to play these drums and percussion instruments was really challenging. I can't begin to tell you the humiliation and injuries to my hands that I have endured over these 6 years. But somehow I hung in there, and by 2006, I was starting to get noticed. I now play drums as a facilitator, where I lead groups sometimes of 200 or more, where I perform on stage, where I hold drumming and spiritual workshops, and where I speak on how drumming can be applied to team-building in business and sports. I'm working on a book on my research and efforts with drumming and the brain.
True shunt matching requires an evaluation of each patient's individual CSF outflow needs, degree of shunt dependency, height, weight, and approximate assessment of ventricular (ICP) and abdominal cavity pressures, and consideration of anticipated growth ( in children). The best known method capable of providing an accurate scientific measurement of each patient's CSF outflow needs and degree of shunt dependency is "pulse wave measurement," which is costly and requires the insertion of one or more needles into the CSF spinal pathway. This technique had been explored by Eldon Foltz, M.D., at the University of California at Irvine in the 1980s. More recently, it was modified and is used commonly by physicians such as Mike Williams, M.D., at John's Hopkins Medical Center, in the evaluation of NPH. Though this procedure has been helpful in confirming NPH and other forms of hydrocephalus, for the full consideration of shunt matching, the physician must still factor in patient height, weight, and approximate abdominal cavity pressures when selecting a specific shunt system.
In consideration, my tandem application of the DiaCeph Test with an in-office ICP shunt tap provided promising results. In this protocol, the neurosurgeon compares DiaCeph Test monitoring to ICP shunt tap findings, recording observations of the patient's status and manometer readings in the supine and upright postures. The neurosurgeon can then reference the information obtained to specifications of various CNS shunts, and comparison bench tests findings in a study by Aschoff, et. al and Colleagues at the University of Heidelberg. The protocol provides some similar information to that of 48 hour in-hospital ICP monitoring, with much less cost and risks. Proper shunt matching and performance is the primary determinant of qualify of life after shunting. Non-medicinal therapies and techniques presented on our site can aid in managing non-surgical complaints associated with hydrocephalus and many other neurological disorders.
There is no reliable database today in the U.S., on CNS shunting outcomes, for the estimated 300,000 to 600,000 persons living with shunted hydrocephalus. Limited patient surveys by the National Hydrocephalus Foundation, the Hydrocephalus Association, and other groups report substantial unresolved quality of life issues. One survey will paint an optimistic picture, then the same organization in another survey will get much less favorable results on nearly identical questions. The Hydrocephalus Association's 1999 patient survey carried out a survey (included on pages 8-11) to present at the STAMP Conference. In cases where hydrocephalus develops in childhood, disability data is often not accurate without employment or residence outside of the home/caregiver setting. In the U.S., it doesn't appear that either PET or fMRI imaging, which could be helpful in understanding chronic changes associated with hydrocephalus, will be widely used due to poor insurance reimbursement. I am unaware of any worldwide epidemiology data on hydrocephalus, made difficult by the unavailability of reporting in underdeveloped countries.
I also present new information in this study Neuro-Compensatory Mechanisms in managing chronic neurological complaints common to hydrocephalus and many other neurological disorders, including, post traumatic stress disorder. This study focused attention on the hippocampus, neuro-hypersensitivities, and learning. My findings, as well as other content on this topic, corresponds with CNN's March 27, 2005 "Memory" Series and complaints raised by neuro-overstimulation and dysfunction of the hippocampus. You can read CNN's Full March 27th Program Transcript here. Our section on Music & Art Therapy provides some valuable techniques in the use music, art, and other methods of biofeedback for compensation of neurological complaints and improving well being. We are authoring a new section on the use of "Drum Circles" for health & wellness, education, and team communication.
Within the sphere of neurosciences research, balance, and cognitive disorders, I published a sensory overload and balance study that identified techniques to help individuals who suffer from these neurological complaints from hydrocephalus, multiple sclerosis, Parkinson's disease, stroke, TBI, migraine, and similar disorders. These disorders tend to share common complaints in memory and concentration, headache, balance, and neuro hypersensitivity. We found new benefits of audible rhythm and drumming in improving patient outcomes.
Assistive Mobile Phone and mHealth Applications will Greatly Benefit those of us with Hydrocephalus
 I believe new mHealth mobile phone apps,
audio and video,
and organizational apps will greatly aid the outlook and independence
of individuals affected by hydrocephalus. My
DiaCeph Test could be made to
run as an app for a mobile phone, but there remain some privacy and
reimbursement hurdles that must first be addressed. Smart phones like the Blackberry Bold
at left (which I have) offer a lot of features and inexpensive apps. I earlier discussed assistive aids in
assistive
technology. Each user,
caregiver, or physician/case manager must determine
which devices, and applications, best suit the needs of each individual.
I believe new mHealth mobile phone apps,
audio and video,
and organizational apps will greatly aid the outlook and independence
of individuals affected by hydrocephalus. My
DiaCeph Test could be made to
run as an app for a mobile phone, but there remain some privacy and
reimbursement hurdles that must first be addressed. Smart phones like the Blackberry Bold
at left (which I have) offer a lot of features and inexpensive apps. I earlier discussed assistive aids in
assistive
technology. Each user,
caregiver, or physician/case manager must determine
which devices, and applications, best suit the needs of each individual.
I encourage visitors to read the updated "Shunt Selection Model." The paper provides neurosurgeons, shunt manufacturers, researchers, families, and patients with insights on our tandem protocol of DiaCeph monitoring with a single in-office ICP assessment - to aid in shunt selection, in finding the best programmable shunt pressure setting, shunt pre-implantation and NPH work-ups, and post discharge monitoring of shunt and ETV procedures. This paper includes discussion, analysis, links, and information on many commonly used shunts, and incorporates comparative test data on common shunts from Dr. Aschoff, et. al. at the University of Heidelberg's hydrocephalus research center.
Patients implanted with VP shunts should be advised that the presence of a CNS shunt and attached catheters can lead to compromised or weakened meridians, the lines of the energy fields that align the body vertically. Symptoms can be related to a poorly functioning shunt, but no necessarily so. It could be part of your body's normal response to "reject" a foreign body. Typical complaints are back and abdomen pains, headaches and shunt site tenderness, irritability, and fatigue. This problem is actually readily correctable with an assessment and adjustment by a practitioner familiar with meridian alignment, though it could require a series of adjustments.
You may contact me thru hydro[at]diaceph[dot]com with questions regarding hydrocephalus consults, drumming events & therapies, or for speaking at a hydrocephalus or brain related event or fundraiser. If you need to speak with me, send me an email with your phone number and I will call you. As of February 28, 2018, I no longer have a separate home office phone.





